如果您无法下载资料,请参考说明:
1、部分资料下载需要金币,请确保您的账户上有足够的金币
2、已购买过的文档,再次下载不重复扣费
3、资料包下载后请先用软件解压,在使用对应软件打开
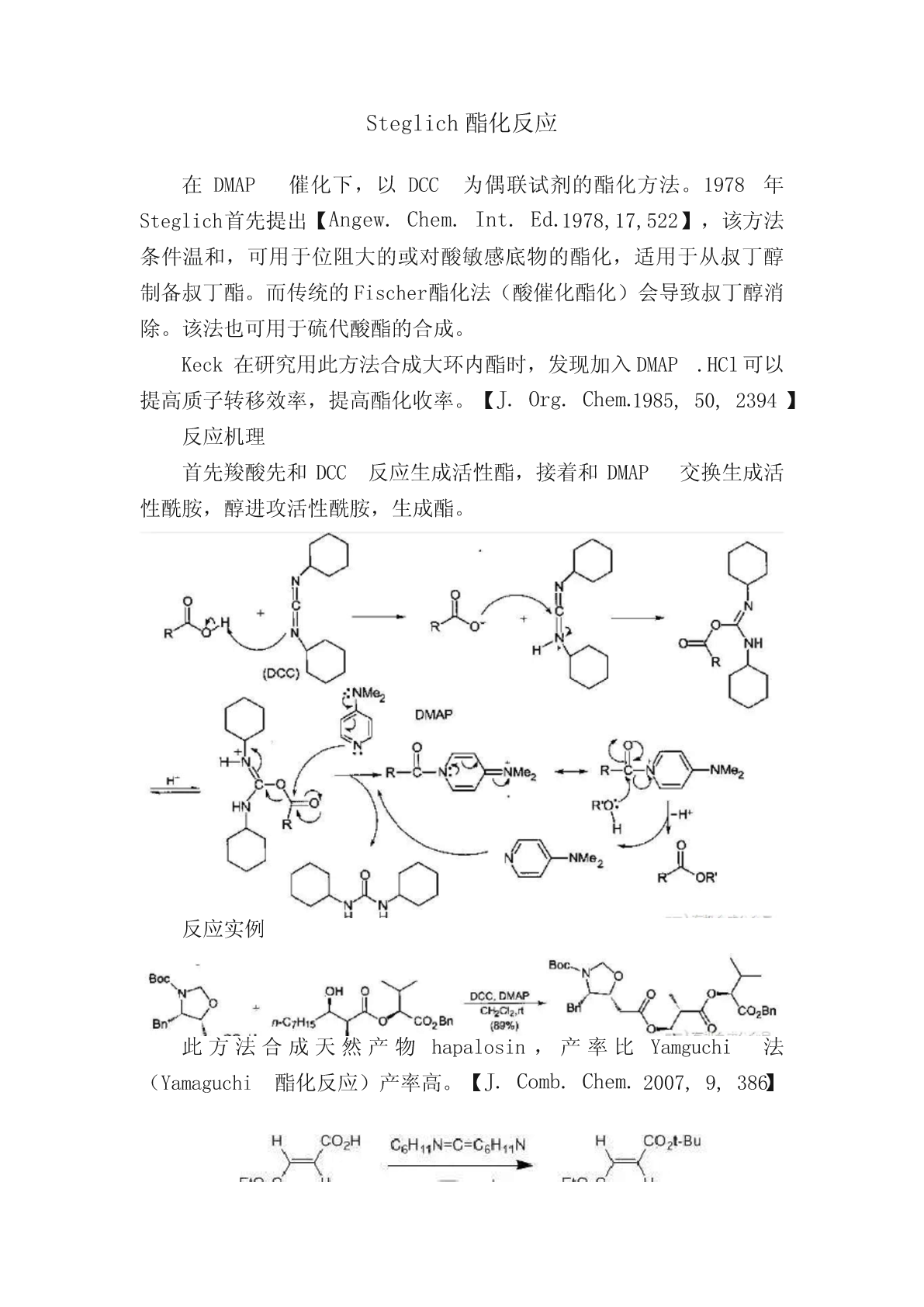
Steglich酯化反应在DMAP催化下,以DCC为偶联试剂的酯化方法。1978年Steglich首先提出【Angew.Chem.Int.Ed.1978,17,522】,该方法条件温和,可用于位阻大的或对酸敏感底物的酯化,适用于从叔丁醇制备叔丁酯。而传统的Fischer酯化法(酸催化酯化)会导致叔丁醇消除。该法也可用于硫代酸酯的合成。Keck在研究用此方法合成大环内酯时,发现加入DMAP.HCl可以提高质子转移效率,提高酯化收率。【J.Org.Chem.1985,50,2394】反应机理首先羧酸先和DCC反应生成活性酯,接着和DMAP交换生成活性酰胺,醇进攻活性酰胺,生成酯。反应实例此方法合成天然产物hapalosin,产率比Yamguchi法(Yamaguchi酯化反应)产率高。【J.Comb.Chem.2007,9,386】A500-mL,one-neckedflaskequippedwithacalciumchloridedryingtubewaschargedwith28.83g(0.20mol)ofmonoethylfumarate,200mLofdrydichloromethane,44.47g(0.60mol)oftert-butylalcohol,and2.00g(0.16mol)of4-dimethylaminopyridine.Thesolutionwasstirredandcooledinanicebathto0°Cwhile45.59g(0.22mol)ofdicyclohexylcarbodiimidewasaddedovera5-minperiod.Afterafurther5minat0°Ctheicebathwasremovedandthedark-brownreactionmixturewasstirredfor3hatroomtemperature.ThedicyclohexylureathathasprecipitatedwasremovedbyfiltrationthroughafrittedBüchnerfunnel(G3),andthefiltratewaswashedwithtwo50-mLportionsof0.5Nhydrochloricacidandtwo50mLportionsofsaturatedsodiumbicarbonatesolution.Duringthisproceduresomeadditionaldicyclohexylureawasprecipitated,whichwasremovedbyfiltrationofbothlayerstofacilitatetheirseparation.Theorganicsolutionwasdriedoveranhydroussodiumsulfateandconcentratedwitharotaryevaporator.Theconcentratewasdistilledunderreducedpressure,affordingafterasmallforerun,30.5–32.5(76–81%)oftert-butylethylfumarate,bp105–107°C(12mm)。【OrganicSyntheses,Coll.Vol.7,p.93(1990);Vol.63,p.183(1985)】编辑自:《有机人名反应、试剂与规则》,黄培强等。