如果您无法下载资料,请参考说明:
1、部分资料下载需要金币,请确保您的账户上有足够的金币
2、已购买过的文档,再次下载不重复扣费
3、资料包下载后请先用软件解压,在使用对应软件打开
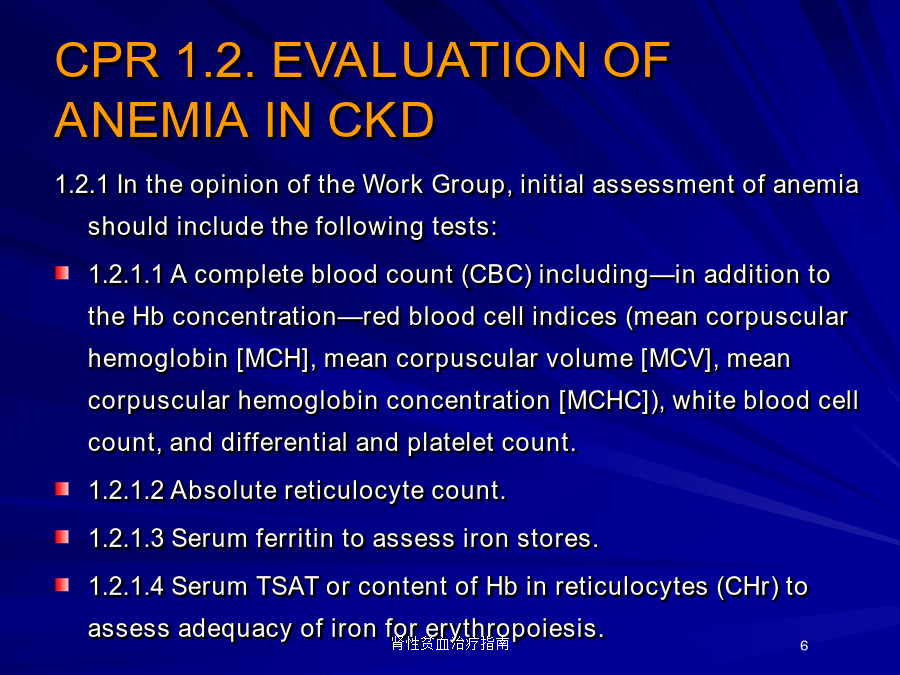
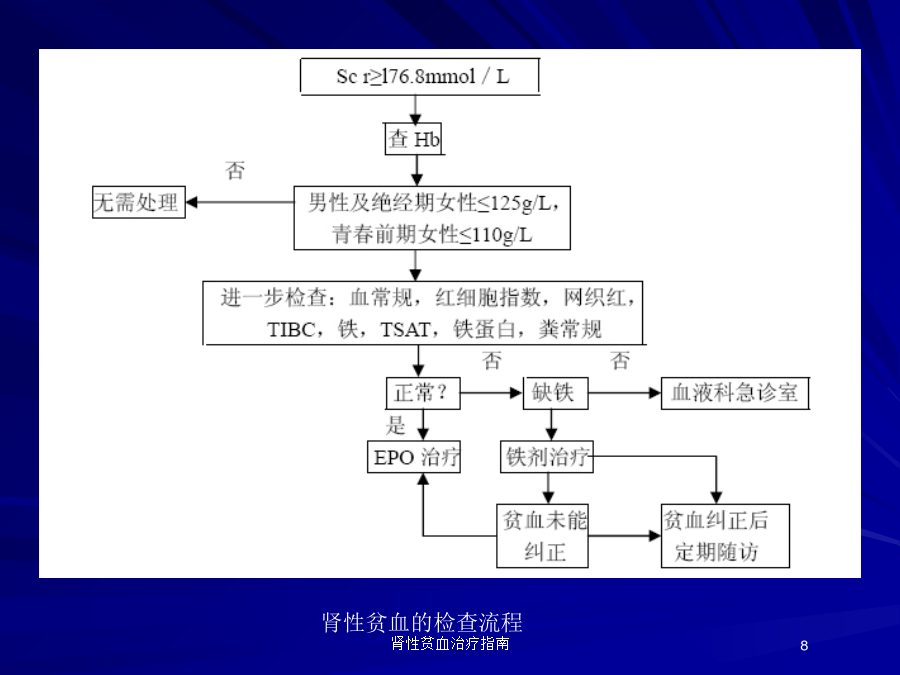
肾性贫血治疗指南CPR1.1.IDENTIFYINGPATIENTSANDINITIATINGEVALUATION贫血定义CPR1.2.EVALUATIONOFANEMIAINCKD贫血实验室检查内容肾性贫血的检查流程CPGANDCPR2.1.HBRANGE2.1HEMOGLOBINTARGET2007rHuEPO治疗肾性贫血靶目标值CPR3.1.USINGESAsCPR3.1.USINGESAs3.1.2ESAdosingCPR3.1.USINGESAsCPR3.1.USINGESAsrHuEPO的临床应用rHuEPO的临床应用rHuEPO的临床应用rHuEPO的临床应用不良反应3.2.USINGIRONAGENTS3.2.USINGIRONAGENTS3.2.USINGIRONAGENTS3.2.USINGIRONAGENTS补充铁剂铁状态评估补充铁剂口服铁剂3.3.USINGPHARMACOLOGICALANDNONPHARMACOLOGICALADJUVANTSTOESATREATMENTINHD-CKD3.4.:TRANSFUSIONTHERAPY3.5.EVALUATINGANDCORRECTINGPERSISTENTFAILURETOREACHORMAINTAININTENDEDHBrHuEPO治疗的低反应性(EPO抵抗)CPR3.5.EVALUATINGANDCORRECTINGPERSISTENTFAILURETOREACHORMAINTAININTENDEDHBrHuEPO抗体介导的纯红细胞再生障碍性贫血(PRCA)长效ESA:持续性促红细胞生成素受体激动剂(continuouserythropoietinreceptoractivator,CERA)Methods.Patientswererandomized(1:1)toreceiveeither1.2g/kgC.E.R.A.Q4WordarbepoetinalfaQW/Q2Wduringa20-weekcorrectionperiodandan8-weekevaluationperiod.Results.TheHbresponserateforC.E.R.A.was94.1%,significantlyhigherthantheprotocol-specified60%responserateandcomparablewithdarbepoetinalfa.C.E.R.A.Q4Wwasnon-inferiortodarbepoetinalfaQW/Q2W,withsimilarmeanHbchangesfrombaselineof1.62g/dLand1.66g/dL,respectively.PatientsreceivingC.E.R.A.showedasteadyriseinHb,withfewerpatientsabovethetargetrangeduringthefirst8weekscomparedwithdarbepoetinalfa.Adverseeventrateswerecomparablebetweenthetreatmentgroups.Conclusion.C.E.R.A.Q4WsuccessfullycorrectsanaemiaandmaintainsstableHblevelswithintherecommendedtargetrangeinnon-dialysisCKDpatients.其他新型制剂红细胞生成素受体激动剂肽治疗纯红细胞再生障碍性贫血谢谢